About Sucraid®
What is Sucraid®?
Sucraid® is the only FDA-approved enzyme replacement therapy indicated for the treatment of genetically determined sucrase deficiency, which is a part of Congenital Sucrase-Isomaltase Deficiency (CSID).
In This Section
Sucraid® is an enzyme replacement therapy that facilitates the breakdown of sucrose (sugar) into monosaccharides for absorption by the small intestine. It has been shown to help alleviate the gastrointestinal (GI) symptoms associated with CSID, and, as a result, patients can maintain a more normal diet that includes sucrose-containing foods.
- For individuals who weigh 33 lb (15 kg) or less, Sucraid® is dispensed in 118-mL translucent plastic bottles, packaged with two bottles per box
- Each mL of solution contains 8,500 international units (IU) of sacrosidase
- A 1-mL measuring scoop is provided with each bottle
- For individuals who weigh more than 33 lb (15 kg), Sucraid® is dispensed in 2-mL single-use containers packaged in a foil pouch
- Each foil pouch includes five single-use containers
- Single-use containers are stable for up to three days at room temperature, 59ºF to 77ºF (15ºC to 25ºC)
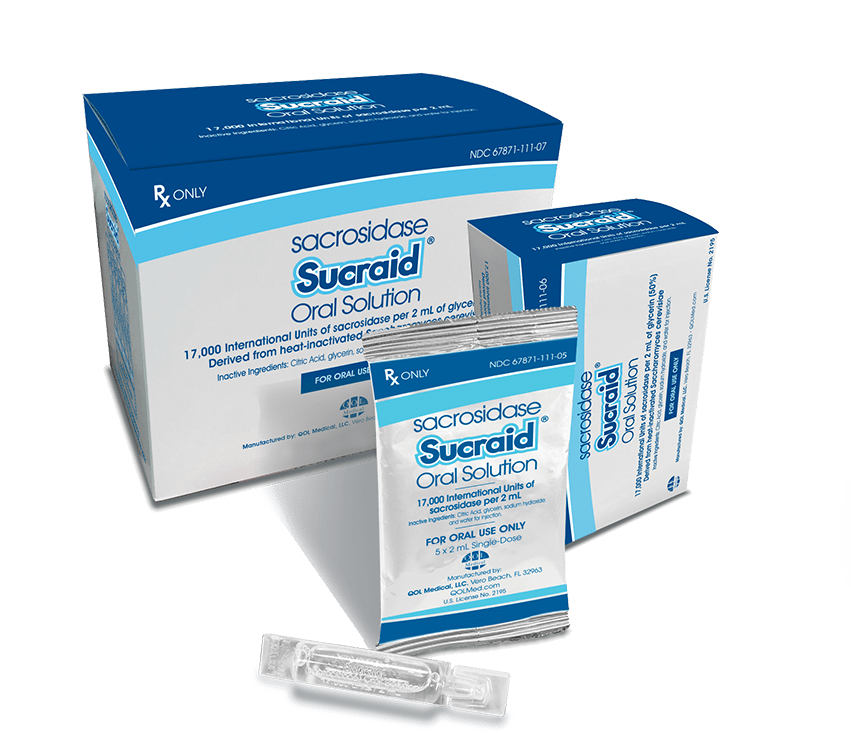
Figure 1. How Sucraid® is dispensed
How Does Sucraid® Work?
Sucraid® has been shown in clinical trials to be effective in the treatment of patients with CSID. The active ingredient in Sucraid® is sacrosidase, a sucrase enzyme replacement that catalyzes the hydrolysis of sucrose into glucose and fructose, thereby facilitating absorption from the small intestine into the bloodstream. Sacrosidase is a potent and robust enzyme; on a per milliliter basis, sacrosidase is approximately 100-fold more potent than endogenous sucrase.1 It has been shown to be stable when stored at 4°C.2
Although Sucraid® provides replacement therapy for deficient sucrase, it does not provide specific replacement therapy for deficient isomaltase.3 Therefore, Sucraid® does not metabolize dietary starches (for example, potato, bread, or pasta) and restricting starch in the diet may still be necessary to minimize symptoms in patients with CSID who are starch intolerant. When starting therapy with Sucraid®, patients who still have symptoms are advised to restrict dietary starch for two weeks and then gradually reintroduce starch while monitoring for gastrointestinal symptoms. Keeping a dietary diary for the first few weeks of therapy is also recommended.
Figure 2. Role of Sucraid® in the metabolism of sucrose and absorption of its metabolic byproducts glucose and fructose across small intestine microvilli
References
- Treem WR, McAdams L, Stanford L, Kastoff G, Justinich C, Hyams J. Sacrosidase therapy for Congenital Sucrase-Isomaltase Deficiency. J Pediatr Gatroenterol Nutr. 1999;28(2):137-142. doi:10.1097/00005176-199902000-00008
- Treem WR, Ahsan N, Sullivan B, et al. Evaluation of liquid yeast-derived sucrase enzyme replacement in patients with sucrase-isomaltase deficiency. Gatroenterology. 1993;105(4):1061-1068. doi:10.1016/0016-5085(93)90950-h
- Sucraid® [package insert]. Vero Beach, FL: QOL Medical, LLC; 2023.


